Bactrim sulfamethoxazole and trimethoprim and Keflex cephalexin are antibiotics prescribed to treat bacterial infections. Cephalexin is a cephalosporin SEF a low spor in antibiotic.
 Sulfa Allergies Overview And What To Avoid
Sulfa Allergies Overview And What To Avoid
Keflex is an antibiotic used to treat infections of the lungs middle ear bones.

Is cephalexin a sulfa drug. The drug sulfasalazine Azulfidine used in the treatment of rheumatoid arthritis Crohns disease and ulcerative colitis. They may also be called sulfa drugs. Is cephalexin a sulfa drug - The QA wiki Yanaorco Archaeological Project Proyecto Arqueologico Yanaorco.
Keflex is a broad-spectrum cephalosporin antibiotic used to treat upper respiratory infections urinary tract infections as well as ear bone and skin infections. Answers 2 Yes cephalexin does contain sulfur. Keflex and Sulfa Allergies Healthfully.
Cephalexin is attributed as antibiotic of cephalosporins group which act against bacteria preventing formation of their cell walls. It works by fighting bacteria in your body. Food and Drug Administration FDA originally approved cephalexin in 1971.
It is used for a wide variety of infections. Cephalosporins are closer to penicillin types of drugs so if you are allergic to penicillins you may have a cross allergy to cephalosporins too. The drug dapsone used to treat dermatitis and some types of pneumonia.
Even if a person is truly allergic to penicillin then they can usually take cephalexin without a reaaction. As one person said the cephalexin is NOT a sulfa drug and there is NO cross reactivity. The information below is based on the dosage guidelines your doctor uses.
If it is Cephalexin you are asking about then no it is not a Sulfa drug. Sulfonamides sulphonamides are a group of man-made synthetic medicines that contain the sulfonamide chemical group. No Keflex is not a sulfa drug.
Cephalexin is used to treat infections caused by bacteria including upper respiratory infections ear infections skin infections urinary tract infections and bone infections. Cephalexin belongs to the class of antibiotics known as cephalosporins. Many people use the term sulfonamide imprecisely to refer only to antibiotics that have a sulfonamide functional group in their chemical structure.
However there are several non-antibiotic. It is a cephalosporin. Please see link below for structure of the molecule.
Cephalexin is a first-generation cephalosporin and is mainly effective against gram-positive bacteria. It is enough resistant to penicillinases of gram positive microorganisms but can be destroyed by beta-lactamases of gram negative ones. Cephalexin is in a class of drugs called cephalosporin antibiotics which work by killing bacteria.
Cephalexin is an antibiotic that is used to treat infections caused by susceptible bacteria. No its completly different chemically and in terms of how it works sulfa drugs inhibit the manufacture of folic acid in the bacteria whereas cephalexin keeps the bacteria from makes a. Some sulfonamides are also devoid of antibacterial activity eg the anticonvulsant sultiame.
Sulfonamide is a functional group a part of a molecule that is the basis of several groups of drugs which are called sulphonamides sulfa drugs or sulpha drugsThe original antibacterial sulfonamides are synthetic nonantibiotic antimicrobial agents that contain the sulfonamide group. People who have a Penicillin allergy may also be allergic to. It is a general antibiotic in the cephalosporin class.
It belongs to the group of antibiotics called fluoroquinolones. Bactrim sulfamethoxazole and trimethoprim is a combination of an antibacterial sulfonamide a sulfa drug and a folic acid inhibitor.
Keflex and Sulfa Allergies Healthfully.

Is keflex a sulfa drug. Keflex is a broad-spectrum cephalosporin antibiotic used to treat upper respiratory infections urinary tract infections as well as ear bone and skin infections. Sulfa drugs include some diabetes medications anti-inflammatory drugs diuretics and more. It is generally used to treat urinary tract infections and respiratory infections.
Cephalexin is a prescription medicine thats used to treat infections caused by bacteria in adults and children at least 1 year old. And for more up-to-date information sign up for our daily newsletter. Levaquin is not a sulfa drug.
Cephalosporins are closer to penicillin types of drugs so if you are allergic to penicillins you may have a cross allergy to cephalosporins too. The National Library of Medicine NLM on the NIH campus in Bethesda Maryland is the worlds largest biomedical library and the developer of electronic information services that delivers data to millions of scientists health professionals and members of the public around the globe every day. It works by fighting bacteria in your body.
No Keflex is not a sulfa drug. Keflex is a cephalosporin antibiotic which is chemically related to a penicillin. Bactrim is a combination of sulfonamide a sulfa drug and a folic acid inhibitor and Keflex is a cephalosporin antibiotic.
No Keflex is not a sulfa drug. People who have a Penicillin allergy may also be allergic to. Common side effects of Bactrim include.
Keflex 250 5ml Dosage - A months worth of pills is available from wholesalers for less than 20. If it is Cephalexin you are asking about then no it is not a Sulfa drug. It is not a sulpha drug and does not contain sulfonamides.
Here well guide you to the very best prices available today. It is a general antibiotic in the cephalosporin class. What Are Possible Side Effects of Bactrim.
Ancef Official FDA information side effects and uses. Keflex is in a group of drugs called cephalosporin antibiotics. Nitrofurantoin is an antibiotic used to treat urinary tract infections UTIs caused by several types of bacteria including E.
Keflex is a cephalosporin antibiotic which is chemically related to a penicillin. Keflex cephalexin is a cephalosporin SEF a low spor in antibiotic. Keflex is used to treat infections caused by bacteria including upper respiratory infections ear infections skin infections urinary tract infections and bone infections.
Kefzol and sulfa allergies - Ablogro - Gazduire bloguri jurnale. Does azithromycin have sulfa in. Similarly persons with an allergy to Keflex have a small risk of also being allergic to penicillins.
It does not contain a sulfa group in its structure so it is not a sulfa drug. It does not contain a sulfa group in its. Sulfonylureas are believed to trigger a cross-reactive allergy in people who are sensitive to other sulfa drugs 3.
Bactrim and Keflex are different types of antibiotics. It is a cephalosporin. It is used for a wide variety of infections.
It should be safe to take if you have a history of sulf. Keflex 250 5ml dosage Best Quality and EXTRA LOW PRICES 250 keflex dosage 5ml. It is a cephalosporin drug which has a small rate of cross-reactivity in persons with a penicillin allergy.
Its often given to treat infections of the skin ears bone.
DUBLIN April 15 2021 PRNewswire -- The Multiple Sclerosis Therapeutics Market Forecast to 2027 - COVID-19 Impact and Global Analysis by Drug. FDA Approves 2 New Multiple Sclerosis Drugs.
 New Drug Candidate For Multiple Sclerosis Patients Dr Makoto Inoue Lab
New Drug Candidate For Multiple Sclerosis Patients Dr Makoto Inoue Lab
16 Zeilen The newest drugs for the treatment of multiple sclerosis include Ponvory Kesimpta Bafiertam.
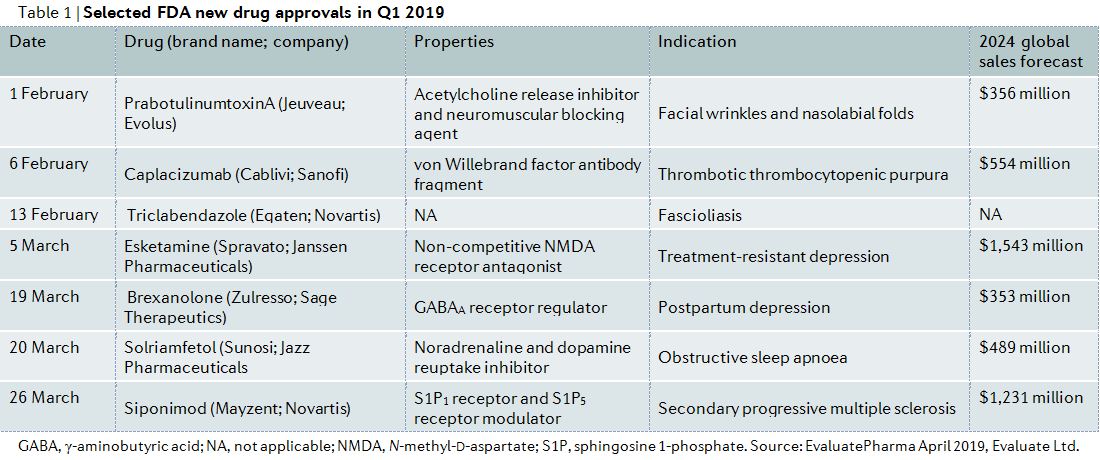
New multiple sclerosis drug. March 29 2019 The US. Biogen has set the. The ABC drugs Avonex Betaseron and Copaxone are often the three first-line agents used for long-term treatment of multiple sclerosis MS.
The MarketWatch News Department was not involved in the creation of this content. Mavenclad is taken for 8 to 10 days in each of the two years of treatment. Gilenya the companys widely used drug for MS treatment.
March 26 2019 The US. If playback doesnt begin shortly try restarting your device. Beta interferon preparations or glatiramer Copaxone may be the initial multiple sclerosis MS therapy chosen by many doctors.
This tablet is taken orally and approved for. The report notes the need for the community to work together to help patients and families address the physical and mental challenges that can result from MS. Food and Drug Administration today approved Mavenclad cladribine tablets to treat relapsing forms of multiple sclerosis MS in adults to include.
About 10 of people with multiple sclerosis are diagnosed with a progressive form primary-progressive MS at the onset of the disease. There are no other treatments. The FDA has approved Vumerity a new drug to treat relapsing forms of multiple sclerosis.
What You Need to Know Mayzent. Multiple Sclerosis Therapeutics Market Analysis - By Drug Class 71 Overview 72 Multiple Sclerosis Therapeutics Market Revenue Share by Drug Class 2019 and 2027. The December 2020 General Social Survey on Patients with Multiple Sclerosis in China by the China Alliance for Rare Diseases found that among people with MS who are unemployed or out of school 90 percent was due to their diagnosis.
Multiple Sclerosis Therapeutics Market Analysis - By Drug Class 71 Overview 72 Multiple Sclerosis Therapeutics Market Revenue Share by Drug Class 2019 and 2027. Experts say Vumerity is effective and has fewer side effects than current medications. Novartis is not new to oral treatments for MS.
76 Zeilen Common medications used to treat multiple sclerosis include Copaxone Gilenya and. New therapies are emerging Siponimod Mayzent was approved by the FDA in 2019. Apr 15 2021 The Expresswire -- The global multiple sclerosis drugs market size is.
Videos you watch may be added to the TVs watch. A clinical trial has begun testing an experimental stem cell treatment against the best available biologic therapies for severe forms of relapsing multiple sclerosis MS. Food and Drug Administration today approved Mayzent siponimod tablets to treat adults with relapsing forms of multiple sclerosis MS to include.
The trial sponsored by the National Institute of Allergy and Infectious Diseases NIAID part of the National Institutes of Health will compare the safety efficacy and cost-effectiveness of the two therapeutic approaches. Studies conducted by the group with mice between 2013 and 2015 had already demonstrated that TnP can treat multiple sclerosis delaying.
Halaman
Renew Physical Therapy
Cari Blog Ini
Label
- 1000
- 10000
- 1500
- 2015
- 29th
- 5000
- abdominal
- ablation
- about
- acetaminophen
- acetonide
- aches
- achy
- acid
- acne
- active
- acupressure
- acupuncture
- adaptogenic
- adderall
- adhd
- admitted
- adults
- advil
- affect
- affordable
- after
- aftercare
- aggregation
- aids
- alcohol
- alcoholic
- aleve
- alienation
- allegra
- allergic
- allergies
- allergy
- allowance
- almond
- american
- aminos
- amiodarone
- anemia
- anise
- ankle
- ankles
- announce
- anorexics
- anti
- antibiotics
- anticoagulant
- antidepressants
- antigen
- anxiety
- anything
- aortic
- apathy
- apple
- arizona
- armpit
- arms
- around
- arterial
- artery
- arthritis
- ascending
- asleep
- aspergers
- assaulted
- atorvastatin
- attack
- attacks
- autism
- autoimmune
- average
- avoid
- awake
- away
- azelastine
- babassu
- babies
- baby
- babymoon
- babysit
- back
- bacterial
- bagel
- baking
- baldness
- balls
- banana
- bars
- base
- basil
- bath
- bathe
- bcaa
- beach
- beans
- beat
- beer
- before
- begin
- behind
- being
- belly
- benadryl
- bench
- benefits
- best
- between
- biceps
- bigger
- bile
- biometric
- biopsy
- bipolar
- birth
- bismol
- bite
- bites
- biting
- black
- blackhead
- bladder
- bleach
- bleeding
- blisters
- bloating
- block
- blockage
- blockers
- blood
- blue
- body
- boiled
- bone
- boneless
- bosley
- bourbon
- bowels
- brace
- bracelet
- braces
- brain
- branch
- break
- breakfast
- breakthrough
- breast
- breastfeed
- breastfeeding
- breath
- breathe
- bridge
- broken
- broth
- brown
- bruise
- bruised
- bruises
- brush
- bubble
- buck
- bugs
- building
- bulk
- bump
- bumps
- bunches
- bunion
- burger
- burn
- burner
- burning
- bursts
- busted
- butter
- caffeine
- calculator
- calorie
- calories
- cambogia
- canal
- cancer
- carb
- carbonated
- carbs
- care
- carnation
- cartilage
- cartoons
- casein
- castor
- castration
- cause
- causes
- causing
- cavities
- cavity
- cell
- cells
- cervical
- cervix
- chadwickâs
- challenge
- chamomile
- chances
- chapped
- charcoal
- check
- cheese
- chemo
- chest
- chicken
- childhood
- children
- chili
- chills
- chin
- chinese
- chipped
- chips
- chocolate
- chronic
- cider
- cigarette
- cirrhosis
- cities
- citric
- clean
- cleaning
- clear
- clinical
- clogged
- clothing
- clots
- cloudy
- coconut
- coffee
- cold
- colic
- colitis
- collar
- cologuard
- colonoscopy
- color
- coloring
- comfortable
- commit
- common
- complex
- concentrate
- conception
- concussion
- condoms
- confirm
- conjunctivitis
- cons
- consequence
- considered
- constipation
- contagious
- contrast
- control
- coolsculpting
- copd
- copper
- corn
- cornstarch
- corona
- cost
- costs
- cottage
- cough
- coughing
- count
- cover
- coverage
- covered
- cramps
- cranberry
- crawl
- cream
- creamer
- crepey
- cries
- crispy
- crown
- crying
- cucumber
- cumin
- curve
- cycle
- cyst
- cystic
- dairy
- damaged
- dandruff
- dark
- dayquil
- days
- dead
- death
- deep
- dehydration
- delt
- dentures
- depressant
- depression
- dermaroller
- description
- desserts
- detachment
- device
- diabetes
- diabetic
- diabeticorum
- diabetics
- diagnosis
- diaper
- diarrhea
- diastolic
- diatomaceous
- didnt
- diet
- diff
- difference
- diovan
- disability
- disc
- discharge
- disease
- dislodge
- disorder
- dizziness
- dizzy
- doctors
- does
- dogs
- donate
- donating
- dosage
- double
- down
- doxycycline
- drainage
- dreams
- dried
- drink
- drinking
- drinks
- drip
- drooling
- drops
- drug
- drumstick
- drunk
- dual
- duct
- dulcolax
- during
- dying
- dynamic
- dysfunction
- early
- ears
- earth
- easy
- eating
- eczema
- educational
- effects
- effexor
- eggs
- ejection
- electric
- electrolysis
- elevation
- eligibility
- elliptical
- emphysema
- endometriosis
- endurance
- energy
- enhancement
- enlarged
- enlargement
- ensure
- epilator
- epilepsy
- epipen
- epsom
- erectile
- espresso
- essential
- estrogen
- eucalyptus
- every
- exactly
- excedrin
- excessive
- exercise
- exercises
- expectancy
- extensions
- extenze
- extraction
- eyeball
- eyebrows
- eyed
- eyelash
- eyelid
- eyelids
- eyes
- face
- facelifts
- facts
- failure
- fake
- fall
- fast
- faster
- fathers
- fatigue
- features
- feed
- feel
- feeling
- feet
- female
- fertility
- fetal
- fever
- fiberglass
- fibroglandular
- fibroids
- fibromyalgia
- fibrosis
- fillers
- fills
- finger
- fingertip
- first
- fish
- fixing
- flapping
- flashes
- flea
- fleas
- flipper
- floor
- flour
- fluocinolone
- fluoride
- foam
- food
- foods
- foot
- forearm
- forehead
- forhead
- formula
- fraction
- freckles
- free
- freeze
- fridge
- fried
- from
- front
- frozen
- fruit
- fungus
- gain
- gainer
- gallon
- games
- garcinia
- garden
- gastritis
- gear
- gender
- generic
- gerd
- germ
- getting
- giant
- gifts
- ginger
- give
- giving
- glass
- gluten
- glycol
- good
- grade
- greasy
- green
- greenish
- grow
- growth
- gummy
- gums
- guys
- hair
- hairline
- hand
- hands
- happens
- hard
- have
- hctz
- head
- headache
- headaches
- headed
- heal
- healed
- healing
- health
- healthiest
- healthy
- Healtline
- hearing
- heart
- heartburn
- heat
- heavy
- heel
- helmets
- help
- hemorrhoids
- henna
- herbs
- hernia
- herniated
- herpes
- high
- hips
- hives
- hold
- holy
- home
- homemade
- homeopathic
- honey
- honeycrisp
- hormonal
- hormone
- hormones
- hospital
- hour
- human
- hurt
- hurts
- hyclate
- hydrochlorothiazide
- hydrocodone
- hydrogen
- hydromorphone
- hydroxycut
- hyperpigmentation
- identical
- idiopathic
- impingement
- implant
- implants
- impotent
- incontinence
- increase
- induce
- induced
- infarction
- infection
- infections
- inflammatory
- info
- information
- ingredient
- ingrown
- inguinal
- inhaler
- injection
- injections
- inner
- insertion
- instant
- instead
- insurance
- internal
- into
- intolerance
- invasive
- invisalign
- iron
- irregular
- isolate
- itching
- itself
- jacket
- jade
- jelly
- jerky
- joint
- juice
- juicing
- just
- juvederm
- kamut
- karo
- keep
- keflex
- keto
- kick
- kidney
- kids
- kill
- killing
- king
- kiwi
- knee
- krispies
- labor
- lactose
- laser
- last
- late
- lead
- leak
- leaky
- learn
- left
- legs
- lemonade
- lesion
- levothyroxin
- lexapro
- lice
- life
- lift
- ligation
- light
- like
- lime
- lipitor
- liposuction
- lipotropic
- lips
- lipstick
- lisinopril
- list
- live
- liver
- living
- lobster
- long
- loose
- losartan
- lose
- loss
- lower
- lumineers
- lump
- lumps
- lunch
- lung
- lying
- lymph
- lymphoma
- macaroni
- made
- make
- making
- male
- many
- mark
- mask
- mass
- massage
- massager
- mattresses
- maximum
- mayo
- mayonnaise
- mcdonalds
- meal
- meals
- mean
- meaning
- meat
- mederma
- medicare
- medication
- medications
- medicine
- meningitis
- meniscus
- menopause
- metabolism
- metastatic
- metformin
- methicillin
- micro
- microblading
- micropigmentation
- middle
- migraine
- migraines
- milk
- minoxidil
- miracle
- miralax
- mirena
- miscarriage
- miso
- missed
- missing
- moisturizer
- mole
- moles
- mons
- month
- months
- morning
- mortality
- mosquito
- most
- mountain
- mouth
- moving
- mozzarella
- much
- mucinex
- mucus
- multiple
- muscle
- muscular
- mushroom
- myocardial
- myself
- nail
- nails
- narcissist
- nasal
- natural
- naturally
- nausea
- near
- neck
- need
- needling
- negative
- nerve
- nerves
- neuropathy
- newborn
- newborns
- nicotine
- night
- nightmares
- nodal
- nodes
- noodles
- normal
- nose
- novolin
- novolog
- nsaids
- numb
- nutrition
- nuts
- nyquil
- oatmeal
- oats
- oblique
- obstruction
- ocean
- often
- oils
- olive
- onion
- open
- opioid
- optimum
- oral
- orange
- origin
- ortho
- osteoarthritis
- outbreak
- outer
- ovarian
- over
- overdose
- ovulating
- oximetry
- pack
- pain
- palmar
- palmetto
- palms
- palpitation
- pancreas
- pancreatic
- papillomavirus
- para
- parental
- parents
- part
- pasta
- patch
- pattern
- pcos
- peanut
- peas
- peeling
- pelvis
- penile
- people
- peppermint
- percocet
- period
- periodontal
- periods
- peroxide
- person
- personality
- peyronies
- phase
- photos
- physical
- pictures
- piercing
- pill
- pillow
- pillows
- pills
- pilonidal
- pimple
- pimples
- pinched
- pineapple
- pink
- plan
- plans
- plantar
- plasma
- plateau
- platelet
- plug
- plugs
- plum
- plus
- pneumonia
- point
- points
- polish
- polyps
- popping
- popular
- pore
- pose
- poses
- position
- possible
- post
- postpartum
- postures
- potassium
- potato
- pound
- pounds
- powder
- powders
- precautions
- prednisone
- pregnancy
- pregnant
- preparation
- presion
- pressure
- preterm
- pretzel
- prevent
- primary
- prince
- proactive
- probiotic
- probiotics
- procardia
- prognosis
- progressive
- propylene
- pros
- protein
- prozac
- psoriasis
- psoriatic
- pubis
- pudding
- pull
- pulled
- pulling
- pulmonary
- pulse
- purified
- qtips
- quick
- quickly
- quinoa
- quitting
- radiating
- ramen
- rapid
- rash
- rashes
- rate
- rates
- rating
- razor
- reaction
- reactive
- rear
- recipe
- recipes
- recover
- recovery
- reduction
- relationship
- relief
- relieve
- remedies
- remedy
- remission
- removal
- remove
- remover
- removing
- renal
- replace
- replacement
- report
- requip
- resistant
- rest
- restaurants
- results
- retainer
- retainers
- reuse
- reversal
- reverse
- review
- reviews
- rheumatoid
- rice
- right
- rinds
- ringing
- risk
- roll
- rolling
- roof
- room
- root
- round
- runners
- running
- safe
- safflower
- saline
- salt
- salts
- sandwich
- save
- scabies
- scale
- scalp
- scan
- scar
- scarring
- scars
- schedule
- schizophrenia
- sclerosis
- scotch
- screening
- sedation
- seeds
- seizure
- sensation
- sensitive
- sensory
- sentence
- septoplasty
- seroquel
- sesame
- sexually
- shaft
- shakes
- sharing
- shave
- shaving
- sheets
- shingles
- shorten
- shortness
- shot
- should
- shoulder
- show
- showing
- shrink
- sick
- sickness
- side
- sign
- signs
- silica
- silicone
- sinus
- sinusitis
- sitting
- size
- skimmed
- skin
- skittles
- skunk
- skyla
- sleep
- sleeping
- sleepy
- small
- smear
- smell
- smells
- smile
- smiling
- smoke
- smoking
- snack
- snap
- snoring
- soap
- soaps
- soba
- sober
- soda
- sodium
- soles
- solids
- solution
- someone
- sonogram
- soon
- sore
- sores
- soup
- sour
- specialist
- specific
- spermicide
- spider
- spinach
- spine
- spit
- splenda
- splinters
- spot
- spots
- spotting
- sprain
- spray
- spread
- stack
- stage
- stages
- stannous
- staphylococcus
- star
- start
- started
- stay
- stds
- steel
- stem
- stenosis
- stevia
- stimulant
- sting
- stings
- stinks
- stitches
- stomach
- stone
- stones
- stool
- stools
- stop
- stopping
- stories
- straight
- strap
- stream
- strength
- strengthening
- strep
- stress
- stretch
- stretches
- strongest
- stuck
- substitute
- subways
- sudafed
- sugar
- sulfa
- sunburn
- supplement
- supplements
- supported
- supposed
- surfaces
- surgery
- surgical
- survival
- swallowing
- sweating
- swedish
- sweet
- swelling
- swimming
- swings
- swiss
- swollen
- symptom
- symptoms
- syrup
- system
- tablespoon
- tags
- tail
- take
- tamanu
- tampon
- tampons
- tansy
- tape
- taping
- taste
- tattoo
- tears
- teen
- teeth
- teething
- tell
- temporary
- tendonitis
- tenodesis
- terbutaline
- term
- terrors
- test
- testicular
- testing
- testis
- testosterone
- tests
- that
- therapy
- thick
- thicker
- thigh
- thighs
- thinners
- third
- throat
- throats
- throbbing
- thrombosis
- throw
- throwing
- thumb
- thyme
- tibial
- ticks
- tied
- tight
- tightness
- time
- timeline
- tired
- tissue
- toddlers
- toenail
- toenails
- tongue
- tonsil
- tonsillectomy
- tonsillitis
- tooth
- toothache
- toothpaste
- topamax
- topical
- torn
- tortilla
- tract
- transplant
- tratamiento
- trazodone
- treat
- treatment
- treatments
- treats
- tree
- trigger
- trimester
- triplet
- truth
- tuck
- tummy
- tuna
- turbinate
- turkey
- twins
- tylenol
- type
- ulcerative
- ultra
- ultrasound
- umbilical
- under
- underwear
- undigested
- unisom
- units
- untreated
- upper
- urinary
- urine
- uterus
- vaccine
- vaginal
- valtrex
- valve
- vapor
- variability
- varicose
- vasectomy
- vegan
- vegans
- vegetable
- vein
- veins
- veneer
- veslcare
- viagra
- vinegar
- vision
- vitals
- vitamin
- vitamins
- vyvanse
- waist
- walk
- wall
- want
- wart
- warts
- wash
- water
- waxing
- ways
- weakness
- wear
- weed
- week
- weeks
- weight
- wendys
- what
- whats
- wheat
- when
- where
- whey
- which
- while
- whip
- whipped
- whipping
- whiskey
- white
- whole
- wild
- will
- wine
- wipe
- wisdom
- with
- within
- without
- women
- womens
- wont
- work
- working
- workout
- works
- worst
- wrinkles
- yawn
- year
- years
- yeast
- yellow
- yoga
- yogurt
- your
- yourself
- zone
- zyrtec
- zzzquil
