DUBLIN April 15 2021 PRNewswire -- The Multiple Sclerosis Therapeutics Market Forecast to 2027 - COVID-19 Impact and Global Analysis by Drug. FDA Approves 2 New Multiple Sclerosis Drugs.
 New Drug Candidate For Multiple Sclerosis Patients Dr Makoto Inoue Lab
New Drug Candidate For Multiple Sclerosis Patients Dr Makoto Inoue Lab
16 Zeilen The newest drugs for the treatment of multiple sclerosis include Ponvory Kesimpta Bafiertam.
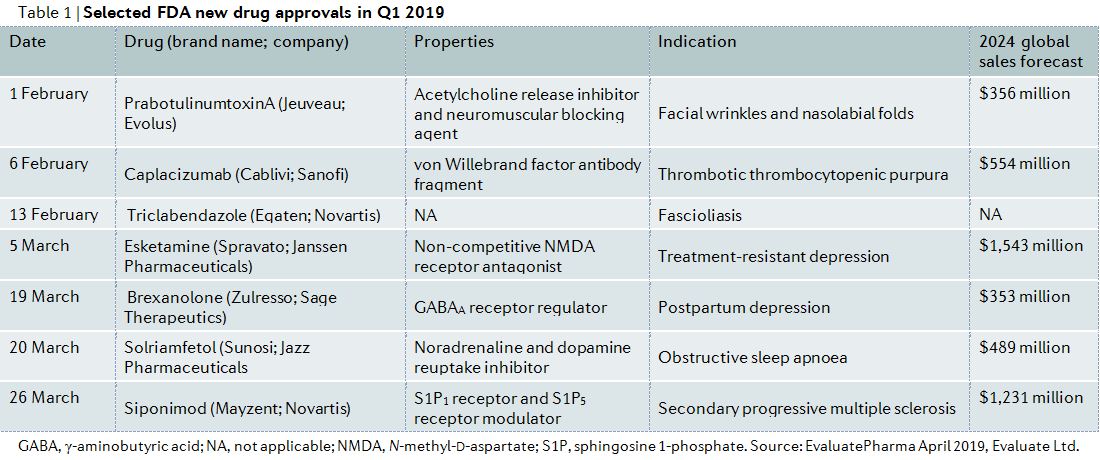
New multiple sclerosis drug. March 29 2019 The US. Biogen has set the. The ABC drugs Avonex Betaseron and Copaxone are often the three first-line agents used for long-term treatment of multiple sclerosis MS.
The MarketWatch News Department was not involved in the creation of this content. Mavenclad is taken for 8 to 10 days in each of the two years of treatment. Gilenya the companys widely used drug for MS treatment.
March 26 2019 The US. If playback doesnt begin shortly try restarting your device. Beta interferon preparations or glatiramer Copaxone may be the initial multiple sclerosis MS therapy chosen by many doctors.
This tablet is taken orally and approved for. The report notes the need for the community to work together to help patients and families address the physical and mental challenges that can result from MS. Food and Drug Administration today approved Mavenclad cladribine tablets to treat relapsing forms of multiple sclerosis MS in adults to include.
About 10 of people with multiple sclerosis are diagnosed with a progressive form primary-progressive MS at the onset of the disease. There are no other treatments. The FDA has approved Vumerity a new drug to treat relapsing forms of multiple sclerosis.
What You Need to Know Mayzent. Multiple Sclerosis Therapeutics Market Analysis - By Drug Class 71 Overview 72 Multiple Sclerosis Therapeutics Market Revenue Share by Drug Class 2019 and 2027. The December 2020 General Social Survey on Patients with Multiple Sclerosis in China by the China Alliance for Rare Diseases found that among people with MS who are unemployed or out of school 90 percent was due to their diagnosis.
Multiple Sclerosis Therapeutics Market Analysis - By Drug Class 71 Overview 72 Multiple Sclerosis Therapeutics Market Revenue Share by Drug Class 2019 and 2027. Experts say Vumerity is effective and has fewer side effects than current medications. Novartis is not new to oral treatments for MS.
76 Zeilen Common medications used to treat multiple sclerosis include Copaxone Gilenya and. New therapies are emerging Siponimod Mayzent was approved by the FDA in 2019. Apr 15 2021 The Expresswire -- The global multiple sclerosis drugs market size is.
Videos you watch may be added to the TVs watch. A clinical trial has begun testing an experimental stem cell treatment against the best available biologic therapies for severe forms of relapsing multiple sclerosis MS. Food and Drug Administration today approved Mayzent siponimod tablets to treat adults with relapsing forms of multiple sclerosis MS to include.
The trial sponsored by the National Institute of Allergy and Infectious Diseases NIAID part of the National Institutes of Health will compare the safety efficacy and cost-effectiveness of the two therapeutic approaches. Studies conducted by the group with mice between 2013 and 2015 had already demonstrated that TnP can treat multiple sclerosis delaying.
Halaman
Renew Physical Therapy
Cari Blog Ini
Label
- 1000
- 10000
- 1500
- 2015
- 29th
- 5000
- abdominal
- ablation
- about
- acetaminophen
- acetonide
- aches
- achy
- acid
- acne
- active
- acupressure
- acupuncture
- adaptogenic
- adderall
- adhd
- admitted
- adults
- advil
- affect
- affordable
- after
- aftercare
- aggregation
- aids
- alcohol
- alcoholic
- aleve
- alienation
- allegra
- allergic
- allergies
- allergy
- allowance
- almond
- american
- aminos
- amiodarone
- anemia
- anise
- ankle
- ankles
- announce
- anorexics
- anti
- antibiotics
- anticoagulant
- antidepressants
- antigen
- anxiety
- anything
- aortic
- apathy
- apple
- arizona
- armpit
- arms
- around
- arterial
- artery
- arthritis
- ascending
- asleep
- aspergers
- assaulted
- atorvastatin
- attack
- attacks
- autism
- autoimmune
- average
- avoid
- awake
- away
- azelastine
- babassu
- babies
- baby
- babymoon
- babysit
- back
- bacterial
- bagel
- baking
- baldness
- balls
- banana
- bars
- base
- basil
- bath
- bathe
- bcaa
- beach
- beans
- beat
- beer
- before
- begin
- behind
- being
- belly
- benadryl
- bench
- benefits
- best
- between
- biceps
- bigger
- bile
- biometric
- biopsy
- bipolar
- birth
- bismol
- bite
- bites
- biting
- black
- blackhead
- bladder
- bleach
- bleeding
- blisters
- bloating
- block
- blockage
- blockers
- blood
- blue
- body
- boiled
- bone
- boneless
- bosley
- bourbon
- bowels
- brace
- bracelet
- braces
- brain
- branch
- break
- breakfast
- breakthrough
- breast
- breastfeed
- breastfeeding
- breath
- breathe
- bridge
- broken
- broth
- brown
- bruise
- bruised
- bruises
- brush
- bubble
- buck
- bugs
- building
- bulk
- bump
- bumps
- bunches
- bunion
- burger
- burn
- burner
- burning
- bursts
- busted
- butter
- caffeine
- calculator
- calorie
- calories
- cambogia
- canal
- cancer
- carb
- carbonated
- carbs
- care
- carnation
- cartilage
- cartoons
- casein
- castor
- castration
- cause
- causes
- causing
- cavities
- cavity
- cell
- cells
- cervical
- cervix
- chadwickâs
- challenge
- chamomile
- chances
- chapped
- charcoal
- check
- cheese
- chemo
- chest
- chicken
- childhood
- children
- chili
- chills
- chin
- chinese
- chipped
- chips
- chocolate
- chronic
- cider
- cigarette
- cirrhosis
- cities
- citric
- clean
- cleaning
- clear
- clinical
- clogged
- clothing
- clots
- cloudy
- coconut
- coffee
- cold
- colic
- colitis
- collar
- cologuard
- colonoscopy
- color
- coloring
- comfortable
- commit
- common
- complex
- concentrate
- conception
- concussion
- condoms
- confirm
- conjunctivitis
- cons
- consequence
- considered
- constipation
- contagious
- contrast
- control
- coolsculpting
- copd
- copper
- corn
- cornstarch
- corona
- cost
- costs
- cottage
- cough
- coughing
- count
- cover
- coverage
- covered
- cramps
- cranberry
- crawl
- cream
- creamer
- crepey
- cries
- crispy
- crown
- crying
- cucumber
- cumin
- curve
- cycle
- cyst
- cystic
- dairy
- damaged
- dandruff
- dark
- dayquil
- days
- dead
- death
- deep
- dehydration
- delt
- dentures
- depressant
- depression
- dermaroller
- description
- desserts
- detachment
- device
- diabetes
- diabetic
- diabeticorum
- diabetics
- diagnosis
- diaper
- diarrhea
- diastolic
- diatomaceous
- didnt
- diet
- diff
- difference
- diovan
- disability
- disc
- discharge
- disease
- dislodge
- disorder
- dizziness
- dizzy
- doctors
- does
- dogs
- donate
- donating
- dosage
- double
- down
- doxycycline
- drainage
- dreams
- dried
- drink
- drinking
- drinks
- drip
- drooling
- drops
- drug
- drumstick
- drunk
- dual
- duct
- dulcolax
- during
- dying
- dynamic
- dysfunction
- early
- ears
- earth
- easy
- eating
- eczema
- educational
- effects
- effexor
- eggs
- ejection
- electric
- electrolysis
- elevation
- eligibility
- elliptical
- emphysema
- endometriosis
- endurance
- energy
- enhancement
- enlarged
- enlargement
- ensure
- epilator
- epilepsy
- epipen
- epsom
- erectile
- espresso
- essential
- estrogen
- eucalyptus
- every
- exactly
- excedrin
- excessive
- exercise
- exercises
- expectancy
- extensions
- extenze
- extraction
- eyeball
- eyebrows
- eyed
- eyelash
- eyelid
- eyelids
- eyes
- face
- facelifts
- facts
- failure
- fake
- fall
- fast
- faster
- fathers
- fatigue
- features
- feed
- feel
- feeling
- feet
- female
- fertility
- fetal
- fever
- fiberglass
- fibroglandular
- fibroids
- fibromyalgia
- fibrosis
- fillers
- fills
- finger
- fingertip
- first
- fish
- fixing
- flapping
- flashes
- flea
- fleas
- flipper
- floor
- flour
- fluocinolone
- fluoride
- foam
- food
- foods
- foot
- forearm
- forehead
- forhead
- formula
- fraction
- freckles
- free
- freeze
- fridge
- fried
- from
- front
- frozen
- fruit
- fungus
- gain
- gainer
- gallon
- games
- garcinia
- garden
- gastritis
- gear
- gender
- generic
- gerd
- germ
- getting
- giant
- gifts
- ginger
- give
- giving
- glass
- gluten
- glycol
- good
- grade
- greasy
- green
- greenish
- grow
- growth
- gummy
- gums
- guys
- hair
- hairline
- hand
- hands
- happens
- hard
- have
- hctz
- head
- headache
- headaches
- headed
- heal
- healed
- healing
- health
- healthiest
- healthy
- Healtline
- hearing
- heart
- heartburn
- heat
- heavy
- heel
- helmets
- help
- hemorrhoids
- henna
- herbs
- hernia
- herniated
- herpes
- high
- hips
- hives
- hold
- holy
- home
- homemade
- homeopathic
- honey
- honeycrisp
- hormonal
- hormone
- hormones
- hospital
- hour
- human
- hurt
- hurts
- hyclate
- hydrochlorothiazide
- hydrocodone
- hydrogen
- hydromorphone
- hydroxycut
- hyperpigmentation
- identical
- idiopathic
- impingement
- implant
- implants
- impotent
- incontinence
- increase
- induce
- induced
- infarction
- infection
- infections
- inflammatory
- info
- information
- ingredient
- ingrown
- inguinal
- inhaler
- injection
- injections
- inner
- insertion
- instant
- instead
- insurance
- internal
- into
- intolerance
- invasive
- invisalign
- iron
- irregular
- isolate
- itching
- itself
- jacket
- jade
- jelly
- jerky
- joint
- juice
- juicing
- just
- juvederm
- kamut
- karo
- keep
- keflex
- keto
- kick
- kidney
- kids
- kill
- killing
- king
- kiwi
- knee
- krispies
- labor
- lactose
- laser
- last
- late
- lead
- leak
- leaky
- learn
- left
- legs
- lemonade
- lesion
- levothyroxin
- lexapro
- lice
- life
- lift
- ligation
- light
- like
- lime
- lipitor
- liposuction
- lipotropic
- lips
- lipstick
- lisinopril
- list
- live
- liver
- living
- lobster
- long
- loose
- losartan
- lose
- loss
- lower
- lumineers
- lump
- lumps
- lunch
- lung
- lying
- lymph
- lymphoma
- macaroni
- made
- make
- making
- male
- many
- mark
- mask
- mass
- massage
- massager
- mattresses
- maximum
- mayo
- mayonnaise
- mcdonalds
- meal
- meals
- mean
- meaning
- meat
- mederma
- medicare
- medication
- medications
- medicine
- meningitis
- meniscus
- menopause
- metabolism
- metastatic
- metformin
- methicillin
- micro
- microblading
- micropigmentation
- middle
- migraine
- migraines
- milk
- minoxidil
- miracle
- miralax
- mirena
- miscarriage
- miso
- missed
- missing
- moisturizer
- mole
- moles
- mons
- month
- months
- morning
- mortality
- mosquito
- most
- mountain
- mouth
- moving
- mozzarella
- much
- mucinex
- mucus
- multiple
- muscle
- muscular
- mushroom
- myocardial
- myself
- nail
- nails
- narcissist
- nasal
- natural
- naturally
- nausea
- near
- neck
- need
- needling
- negative
- nerve
- nerves
- neuropathy
- newborn
- newborns
- nicotine
- night
- nightmares
- nodal
- nodes
- noodles
- normal
- nose
- novolin
- novolog
- nsaids
- numb
- nutrition
- nuts
- nyquil
- oatmeal
- oats
- oblique
- obstruction
- ocean
- often
- oils
- olive
- onion
- open
- opioid
- optimum
- oral
- orange
- origin
- ortho
- osteoarthritis
- outbreak
- outer
- ovarian
- over
- overdose
- ovulating
- oximetry
- pack
- pain
- palmar
- palmetto
- palms
- palpitation
- pancreas
- pancreatic
- papillomavirus
- para
- parental
- parents
- part
- pasta
- patch
- pattern
- pcos
- peanut
- peas
- peeling
- pelvis
- penile
- people
- peppermint
- percocet
- period
- periodontal
- periods
- peroxide
- person
- personality
- peyronies
- phase
- photos
- physical
- pictures
- piercing
- pill
- pillow
- pillows
- pills
- pilonidal
- pimple
- pimples
- pinched
- pineapple
- pink
- plan
- plans
- plantar
- plasma
- plateau
- platelet
- plug
- plugs
- plum
- plus
- pneumonia
- point
- points
- polish
- polyps
- popping
- popular
- pore
- pose
- poses
- position
- possible
- post
- postpartum
- postures
- potassium
- potato
- pound
- pounds
- powder
- powders
- precautions
- prednisone
- pregnancy
- pregnant
- preparation
- presion
- pressure
- preterm
- pretzel
- prevent
- primary
- prince
- proactive
- probiotic
- probiotics
- procardia
- prognosis
- progressive
- propylene
- pros
- protein
- prozac
- psoriasis
- psoriatic
- pubis
- pudding
- pull
- pulled
- pulling
- pulmonary
- pulse
- purified
- qtips
- quick
- quickly
- quinoa
- quitting
- radiating
- ramen
- rapid
- rash
- rashes
- rate
- rates
- rating
- razor
- reaction
- reactive
- rear
- recipe
- recipes
- recover
- recovery
- reduction
- relationship
- relief
- relieve
- remedies
- remedy
- remission
- removal
- remove
- remover
- removing
- renal
- replace
- replacement
- report
- requip
- resistant
- rest
- restaurants
- results
- retainer
- retainers
- reuse
- reversal
- reverse
- review
- reviews
- rheumatoid
- rice
- right
- rinds
- ringing
- risk
- roll
- rolling
- roof
- room
- root
- round
- runners
- running
- safe
- safflower
- saline
- salt
- salts
- sandwich
- save
- scabies
- scale
- scalp
- scan
- scar
- scarring
- scars
- schedule
- schizophrenia
- sclerosis
- scotch
- screening
- sedation
- seeds
- seizure
- sensation
- sensitive
- sensory
- sentence
- septoplasty
- seroquel
- sesame
- sexually
- shaft
- shakes
- sharing
- shave
- shaving
- sheets
- shingles
- shorten
- shortness
- shot
- should
- shoulder
- show
- showing
- shrink
- sick
- sickness
- side
- sign
- signs
- silica
- silicone
- sinus
- sinusitis
- sitting
- size
- skimmed
- skin
- skittles
- skunk
- skyla
- sleep
- sleeping
- sleepy
- small
- smear
- smell
- smells
- smile
- smiling
- smoke
- smoking
- snack
- snap
- snoring
- soap
- soaps
- soba
- sober
- soda
- sodium
- soles
- solids
- solution
- someone
- sonogram
- soon
- sore
- sores
- soup
- sour
- specialist
- specific
- spermicide
- spider
- spinach
- spine
- spit
- splenda
- splinters
- spot
- spots
- spotting
- sprain
- spray
- spread
- stack
- stage
- stages
- stannous
- staphylococcus
- star
- start
- started
- stay
- stds
- steel
- stem
- stenosis
- stevia
- stimulant
- sting
- stings
- stinks
- stitches
- stomach
- stone
- stones
- stool
- stools
- stop
- stopping
- stories
- straight
- strap
- stream
- strength
- strengthening
- strep
- stress
- stretch
- stretches
- strongest
- stuck
- substitute
- subways
- sudafed
- sugar
- sulfa
- sunburn
- supplement
- supplements
- supported
- supposed
- surfaces
- surgery
- surgical
- survival
- swallowing
- sweating
- swedish
- sweet
- swelling
- swimming
- swings
- swiss
- swollen
- symptom
- symptoms
- syrup
- system
- tablespoon
- tags
- tail
- take
- tamanu
- tampon
- tampons
- tansy
- tape
- taping
- taste
- tattoo
- tears
- teen
- teeth
- teething
- tell
- temporary
- tendonitis
- tenodesis
- terbutaline
- term
- terrors
- test
- testicular
- testing
- testis
- testosterone
- tests
- that
- therapy
- thick
- thicker
- thigh
- thighs
- thinners
- third
- throat
- throats
- throbbing
- thrombosis
- throw
- throwing
- thumb
- thyme
- tibial
- ticks
- tied
- tight
- tightness
- time
- timeline
- tired
- tissue
- toddlers
- toenail
- toenails
- tongue
- tonsil
- tonsillectomy
- tonsillitis
- tooth
- toothache
- toothpaste
- topamax
- topical
- torn
- tortilla
- tract
- transplant
- tratamiento
- trazodone
- treat
- treatment
- treatments
- treats
- tree
- trigger
- trimester
- triplet
- truth
- tuck
- tummy
- tuna
- turbinate
- turkey
- twins
- tylenol
- type
- ulcerative
- ultra
- ultrasound
- umbilical
- under
- underwear
- undigested
- unisom
- units
- untreated
- upper
- urinary
- urine
- uterus
- vaccine
- vaginal
- valtrex
- valve
- vapor
- variability
- varicose
- vasectomy
- vegan
- vegans
- vegetable
- vein
- veins
- veneer
- veslcare
- viagra
- vinegar
- vision
- vitals
- vitamin
- vitamins
- vyvanse
- waist
- walk
- wall
- want
- wart
- warts
- wash
- water
- waxing
- ways
- weakness
- wear
- weed
- week
- weeks
- weight
- wendys
- what
- whats
- wheat
- when
- where
- whey
- which
- while
- whip
- whipped
- whipping
- whiskey
- white
- whole
- wild
- will
- wine
- wipe
- wisdom
- with
- within
- without
- women
- womens
- wont
- work
- working
- workout
- works
- worst
- wrinkles
- yawn
- year
- years
- yeast
- yellow
- yoga
- yogurt
- your
- yourself
- zone
- zyrtec
- zzzquil
